 |
 |
 |
|
Development of the Atom!
|
 |
|
|
 |
 |
 |
 |
 |
 |
Millikan
Millikan was able to measure the charge of an electron from his experiment
called the Oil-Drop experiment in 1909. Millikan was able to try and achieve the measurement of an individual subatomic particle.
Using an atomiser Millikan produced oil droplets above two corresponding plates. The droplets would fall through a hole that
was in the upper plate and be in the space between the plates.You were able to see the oil through a short focal distance
telescope at one side. They were able to get the charge by X-raying, or the droplets could electrify themselves when they
were atomised. Using this experiment he was able to figure out the electrons charge.
Definition
atomiser-(a dispenser that turns a liquid into a fine mist)
During this time the Wright brothers were inventing the airplane, and the first motor powered flight.
Ernest Rutherford
He was first known
for creating alpha, beta, and gamma rays. Rutherford deflected alpha rays with magnetic fields in 1903. He also examined the
intensity of radioactivity and performed the gold foil alpha particle scattering experiment. This experiment created
a new model of the atom called the planetary model. With this experiment he suggested that the atom was nuclear and had
a nucleus. He also proposed that simple rays were obtained through hydrogen and are the positively charged particles of the
proton. After this in 1917 Rutherford put alpha particles through nitrogen gas and observed the sparks of hydrogen and came
to the conclusion the alpha particles were knocking protons out of nitrogen atoms studying nuclear reactions.
During this time World War I was occuring.
|
 |
 |
 |
 |
|
 |
|
|
|
 |
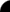 |
 |
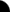 |
 |

Niels Bohr
In 1913 Bohr studied the atoms structure. Bohr recognized that electrons
were at set levels of energy at different distance from the nucleus. He said as long as the electrons were at the right level
then they are steady, but if the electrons change energy levels the energy is either given or taken away.He discovered that
the nucleus is surronded by electron shells that is made up of electrons that orbit the nucleus at different levels. Electrons
with more energy were on the outer shells and the ones with less energy were on the inner shells. Electrons could not live
in between.
During this time the first moving assmebly line began and the one millionth Model T car was finished due to the assembly
line.
|
 |
 |
 |
 |
 |
|
|
|
|
 |
|
Chemistry
ATOMIC DEVELOPMENT
|
|
